CRISPR technology, a revolutionary gene-editing tool, has rapidly transformed scientific landscapes. Its precise ability to alter DNA sequences holds immense promise across diverse fields, from treating genetic diseases to developing novel agricultural products and diagnostics. This powerful technology, however, also presents significant ethical considerations and challenges that demand careful consideration.
The CRISPR-Cas system, derived from a bacterial defense mechanism, utilizes a guide RNA molecule to direct a Cas enzyme to a specific DNA location. This allows for precise gene editing, including gene knockout, insertion, or correction. Different Cas enzymes offer varying functionalities and target specificities, expanding the versatility of this technology.
CRISPR Mechanism of Action
CRISPR-Cas systems are revolutionary gene-editing tools derived from a bacterial defense mechanism against viruses. Understanding their mechanism is crucial for appreciating their potential and limitations in various applications, from treating genetic diseases to developing new agricultural technologies. The system relies on a precise molecular interaction between a guide RNA molecule and a target DNA sequence within the genome.
The CRISPR-Cas system’s core components are the Cas enzyme, guide RNA (gRNA), and the target DNA. The gRNA, a short RNA molecule engineered to be complementary to a specific DNA sequence, acts as a guide, directing the Cas enzyme to the target location in the genome. The Cas enzyme, a nuclease, is responsible for cutting the DNA at the targeted site. This cut initiates the cell’s DNA repair mechanisms, which can be exploited to introduce specific genetic changes.
Cas Enzymes and Their Variations
Several types of Cas enzymes exist, each with unique properties and functionalities. Cas9, the most widely used enzyme, creates a double-stranded break (DSB) in the target DNA. Cas12a (Cpf1), in contrast, creates staggered DSBs and requires a different gRNA structure. Cas13, on the other hand, targets RNA rather than DNA, making it useful for gene regulation and diagnostics. The choice of Cas enzyme depends on the specific application and the desired outcome. For example, Cas9’s simplicity and efficiency have made it a workhorse in many applications, while Cas12a’s different cut characteristics offer advantages in certain contexts. The development of new Cas enzymes continues to expand the capabilities of CRISPR technology.
Guide RNA and Target DNA Specificity
The specificity of CRISPR-mediated gene editing is primarily determined by the sequence of the gRNA. The gRNA must be designed to precisely match the target DNA sequence, ensuring that the Cas enzyme cuts only at the intended location. A crucial aspect of gRNA design is minimizing off-target effects – unintended cuts at similar sequences elsewhere in the genome. Computational tools and careful experimental validation are essential to ensure the accuracy and safety of CRISPR-mediated gene editing. Mismatch tolerance varies between different Cas enzymes and can affect the specificity of targeting.
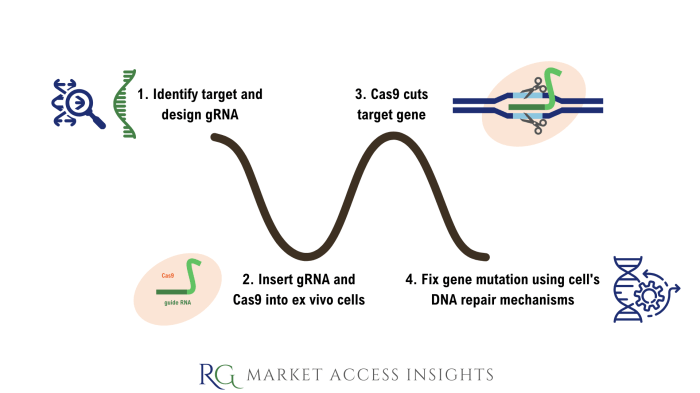
CRISPR-Mediated Gene Editing: A Flowchart
The process of CRISPR-mediated gene editing can be visualized as a series of steps:
1. gRNA Design and Synthesis: A gRNA molecule is designed to target a specific DNA sequence.
2. Cas Enzyme Delivery: The Cas enzyme and gRNA are delivered into the target cell (e.g., using viral vectors or lipid nanoparticles).
3. Target DNA Recognition: The gRNA guides the Cas enzyme to the target DNA sequence.
4. DNA Cleavage: The Cas enzyme creates a double-stranded break (DSB) in the target DNA.
5. DNA Repair: The cell’s DNA repair mechanisms are activated. This can lead to either non-homologous end joining (NHEJ), which often introduces insertions or deletions (indels), or homology-directed repair (HDR), which allows for precise gene replacement using a provided DNA template.
6. Gene Editing Outcome: The outcome depends on the DNA repair pathway utilized and the presence of a repair template. NHEJ typically results in gene disruption, while HDR allows for precise gene modification.
Applications in Gene Therapy: Crispr Technology
CRISPR-Cas9 technology, with its precision and relative ease of use, has rapidly emerged as a leading gene editing tool with significant potential in gene therapy. Its ability to target and modify specific DNA sequences offers the possibility of correcting genetic defects responsible for a wide range of inherited diseases. However, the transition from bench research to successful clinical application presents considerable challenges.
CRISPR-Based Gene Therapy Trials for Inherited Diseases
Several clinical trials are underway exploring CRISPR’s therapeutic potential for inherited diseases. Examples include trials targeting β-thalassemia and sickle cell disease, both blood disorders caused by mutations in the genes responsible for hemoglobin production. These trials involve modifying hematopoietic stem cells (cells that give rise to blood cells) ex vivo (outside the body) to correct the genetic defect before reintroducing them into the patient. Other ongoing trials are investigating CRISPR’s application in treating inherited blindness, muscular dystrophy, and various types of cancer. These trials represent a significant step towards realizing the therapeutic promise of CRISPR technology, although long-term efficacy and safety data are still being gathered.
Challenges and Limitations of CRISPR in Gene Therapy
Despite its promise, CRISPR-based gene therapy faces several challenges. Off-target effects, where CRISPR edits unintended parts of the genome, remain a major concern. These unintended edits could have unpredictable and potentially harmful consequences. Delivering the CRISPR-Cas9 system efficiently and specifically to the target cells or tissues in the body is another significant hurdle. Viral vectors, often used for delivery, can trigger immune responses or have limited targeting capabilities. Furthermore, the complexity and cost of developing and manufacturing CRISPR-based therapies pose significant barriers to widespread access. Finally, achieving sustained therapeutic effects can be challenging, as the edited cells may not always survive or proliferate effectively.
Ethical Considerations Surrounding CRISPR Gene Therapy
The potential of CRISPR to alter the human germline (the genes passed from parents to offspring) raises profound ethical concerns. Germline editing could have unpredictable and irreversible consequences for future generations, and the potential for misuse raises concerns about eugenics and social equity. Furthermore, ensuring informed consent from patients and addressing potential biases in access to these therapies are crucial ethical considerations. The debate surrounding germline editing highlights the need for careful regulation and ethical oversight to guide the development and application of CRISPR technology in gene therapy.
Comparison of CRISPR with Other Gene Editing Techniques
| Technique | Efficacy | Safety Profile | Applications |
|---|---|---|---|
| CRISPR-Cas9 | High; Precise targeting but off-target effects are a concern | Moderate; Off-target effects and immune responses are potential risks | Wide range of applications, including gene therapy for inherited diseases and cancer |
| ZFNs (Zinc Finger Nucleases) | Moderate; Less precise than CRISPR | Moderate; Off-target effects are a concern | Limited applications compared to CRISPR |
| TALENs (Transcription Activator-Like Effector Nucleases) | Moderate; Less precise than CRISPR | Moderate; Off-target effects are a concern | Limited applications compared to CRISPR |
| Base Editing | High; Precise base changes without double-strand breaks | High; Reduced risk of off-target effects | Emerging applications in gene therapy |
Applications in Agriculture
CRISPR-Cas9 gene editing technology offers unprecedented opportunities to improve crop production and address global food security challenges. By precisely modifying the genomes of plants, researchers can enhance desirable traits, leading to increased yields, enhanced nutritional value, and improved resistance to pests, diseases, and environmental stresses. This section will explore the applications of CRISPR in agriculture, highlighting both the potential benefits and the associated risks and regulatory considerations.
Examples of CRISPR Applications in Crop Improvement
CRISPR has been successfully applied to a wide range of crops to improve various traits. For instance, researchers have used CRISPR to enhance disease resistance in rice by targeting genes involved in susceptibility to bacterial blight. Similarly, CRISPR has been employed to improve the nutritional content of crops, such as increasing the vitamin content in tomatoes or enhancing the protein content in wheat. Yield enhancement is another key application; CRISPR can be used to modify genes controlling plant architecture, leading to more efficient light capture and ultimately higher yields. For example, modifications to flowering time can optimize crop growth for specific environments. Furthermore, CRISPR can be used to improve a plant’s tolerance to herbicides or environmental stresses like drought or salinity.
Potential Benefits and Risks of Using CRISPR in Agriculture
The potential benefits of CRISPR-based crop improvement are substantial. Increased crop yields can contribute significantly to global food security, particularly in regions facing food shortages. Enhanced nutritional value can improve public health by increasing the intake of essential vitamins and minerals. Improved resistance to pests and diseases can reduce reliance on pesticides and herbicides, minimizing environmental impact. However, there are also potential risks associated with CRISPR technology in agriculture. Off-target effects, where unintended genetic modifications occur, are a concern. The potential for the development of herbicide-resistant weeds or the unintended transfer of modified genes to wild relatives are also risks that need careful consideration and mitigation strategies. Furthermore, there are ethical concerns surrounding the release of genetically modified organisms (GMOs) into the environment and the potential impact on biodiversity.
Potential Regulatory Hurdles for CRISPR-Edited Crops
The regulatory landscape for CRISPR-edited crops varies significantly across different countries. In some regions, CRISPR-edited crops are subject to the same stringent regulations as traditionally bred GMOs, while in others, a more lenient approach is adopted, distinguishing between CRISPR-edited crops that could have arisen through traditional breeding methods and those with more significant genetic alterations. This regulatory uncertainty can create significant hurdles for the development and commercialization of CRISPR-edited crops, leading to increased costs and delays. Harmonizing regulatory frameworks internationally is crucial to facilitate the adoption of this technology and realize its full potential for improving agriculture.
CRISPR-Edited Crops Currently Under Development or Commercialization
Several CRISPR-edited crops are currently under development or have already reached commercialization. These include disease-resistant rice, wheat with enhanced protein content, and tomatoes with improved shelf life. The specific examples and the stage of development vary depending on the crop and the country. Detailed information on specific products is readily available from various research institutions and agricultural biotechnology companies, although a comprehensive, constantly updated list is difficult to maintain due to the rapidly evolving nature of the field. A few examples include various lines of corn, soybean, and potato undergoing field trials or early commercial release with enhanced pest resistance or improved nutritional profile. The pace of development is rapid, with new applications and products emerging regularly.
Applications in Diagnostics
CRISPR-Cas systems, renowned for their gene-editing capabilities, have emerged as powerful tools in diagnostics, offering rapid, sensitive, and cost-effective methods for detecting various pathogens and genetic mutations. Their ability to specifically target and cleave DNA or RNA makes them ideal for developing diagnostic assays with high accuracy and speed.
CRISPR-based diagnostics leverage the guided nuclease activity of CRISPR-Cas systems to detect target nucleic acid sequences. The presence or absence of the target sequence is then translated into a detectable signal, indicating the presence or absence of a pathogen or genetic mutation. This contrasts sharply with traditional methods, which often require extensive sample preparation, multiple steps, and longer turnaround times.
Rapid and Sensitive Pathogen Detection using CRISPR
CRISPR-Cas systems, particularly Cas12 and Cas13, are at the forefront of rapid pathogen detection. Cas12, a type V CRISPR-associated enzyme, exhibits collateral cleavage activity after recognizing its target DNA. This means that upon binding to its target, Cas12 nonspecifically cleaves any single-stranded DNA in the vicinity, generating a detectable signal. Similarly, Cas13, a type VI CRISPR-associated enzyme, exhibits collateral cleavage activity after recognizing its target RNA. This collateral activity is harnessed in diagnostic assays to amplify the signal, resulting in highly sensitive detection even with low amounts of target nucleic acid. For example, SHERLOCK (Specific High-sensitivity Enzymatic Reporter unlocking) and DETECTR (DNA Endonuclease Targeted CRISPR Trans Reporter) are two prominent CRISPR-based diagnostic platforms that utilize this principle for rapid and sensitive detection of various viruses, bacteria, and other pathogens. These systems have demonstrated remarkable sensitivity, capable of detecting even a single copy of viral RNA or DNA.
Advantages of CRISPR-based Diagnostics over Traditional Methods
CRISPR-based diagnostics offer several advantages over traditional methods like PCR (Polymerase Chain Reaction) and ELISA (Enzyme-Linked Immunosorbent Assay). Firstly, CRISPR assays often require less complex sample preparation and fewer steps, reducing the overall assay time significantly. Secondly, CRISPR systems can be designed to target specific sequences with high specificity, minimizing the risk of false positives or negatives. Thirdly, CRISPR-based diagnostics can be designed to be portable and point-of-care, enabling rapid testing in resource-limited settings. Finally, the cost of CRISPR-based diagnostics is potentially lower than that of some traditional methods, making them more accessible.
Development Process of a CRISPR-based Diagnostic Test for a Specific Disease
Developing a CRISPR-based diagnostic test involves several key steps. First, the target nucleic acid sequence specific to the pathogen or disease needs to be identified. Next, a CRISPR-Cas system (e.g., Cas12 or Cas13) is selected, and a guide RNA (gRNA) is designed to target the specific sequence. Then, a reporter system is incorporated to generate a detectable signal upon target recognition. This could involve fluorescent reporters, electrochemical signals, or lateral flow assays. The entire assay is then optimized for sensitivity, specificity, and speed. Rigorous testing and validation are crucial to ensure the accuracy and reliability of the diagnostic test before clinical implementation. For instance, the development of a CRISPR-based diagnostic for influenza would involve identifying conserved regions within the influenza genome, designing a gRNA to target these regions, and coupling the Cas enzyme with a reporter system to visually detect the presence of influenza viral RNA.
Comparison of CRISPR-based Diagnostics with Other Molecular Diagnostic Techniques
CRISPR-based diagnostics are compared favorably to other molecular diagnostic techniques like PCR and microarrays. While PCR offers high sensitivity and specificity, it often requires specialized equipment and trained personnel. Microarrays, although capable of detecting multiple targets simultaneously, can be expensive and require sophisticated equipment. CRISPR-based diagnostics offer a balance between sensitivity, specificity, speed, and cost-effectiveness, making them a promising alternative for various applications, particularly in point-of-care diagnostics and resource-limited settings. However, challenges remain in terms of standardization and regulatory approval for widespread clinical use.
Off-Target Effects and Safety Concerns
CRISPR-Cas systems, while revolutionary, are not without their challenges. The inherent risk of off-target effects, where the system modifies unintended genomic locations, poses a significant hurdle to their widespread clinical and agricultural application. Understanding these potential issues and developing strategies to mitigate them is crucial for responsible and safe implementation of this powerful technology.
The precision of CRISPR-Cas systems, while generally high, is not perfect. The guide RNA, designed to target a specific DNA sequence, can sometimes bind to other sequences with similar, albeit not identical, sequences. This can lead to unintended edits in the genome, potentially resulting in harmful mutations or disrupting essential genes. The probability of off-target effects varies depending on several factors, including the guide RNA design, the target sequence itself, and the specific CRISPR-Cas enzyme used. Furthermore, the delivery method of the CRISPR components can also influence the frequency of off-target events.
Minimizing Off-Target Editing
Several strategies are employed to minimize off-target editing. Improved guide RNA design algorithms utilize sophisticated computational tools to predict and avoid potential off-target sites. These algorithms consider factors like the number of mismatches between the guide RNA and potential off-target sites, the position of these mismatches, and the overall thermodynamic stability of the guide RNA-DNA complex. Furthermore, high-fidelity Cas enzymes, engineered variants of the naturally occurring enzymes, have been developed with improved target specificity. These engineered nucleases show a reduced propensity to bind and cleave off-target sites while maintaining high on-target activity. Additionally, techniques like paired nickases, which require two guide RNAs to create a double-strand break, enhance specificity by requiring simultaneous binding of two guide RNAs at the target site, significantly reducing off-target activity.
Safety Testing for CRISPR-Based Therapies and Applications
Rigorous safety testing is essential before any CRISPR-based therapy or application can be considered for clinical use or widespread deployment. These tests involve a multi-layered approach. Initially, in vitro assays using cell lines are performed to assess the on-target activity and frequency of off-target events. Next, in vivo studies in animal models are conducted to evaluate the efficacy and safety of the CRISPR-based intervention in a more complex biological system. These studies meticulously assess potential off-target effects, immune responses, and overall toxicity. Comprehensive genomic sequencing is employed to identify any unintended genomic alterations. Only after successful completion of these pre-clinical studies can CRISPR-based therapies proceed to human clinical trials, which involve further rigorous safety monitoring and evaluation. For agricultural applications, similar safety assessments are conducted, focusing on potential impacts on the environment and the long-term consequences of genetic modifications.
Strategies for Improving Specificity of CRISPR-Cas Systems
The development of more precise CRISPR systems is an active area of research. Here are several strategies currently being pursued:
- Improved guide RNA design algorithms: The development of more sophisticated algorithms that can accurately predict and avoid potential off-target sites is crucial.
- High-fidelity Cas enzymes: Engineered Cas enzymes with improved target specificity reduce the likelihood of off-target editing.
- Paired nickases: Using two guide RNAs increases the precision of the system by requiring simultaneous binding at the target site.
- Base editors: These tools allow for targeted base changes without causing double-strand breaks, minimizing the risk of off-target effects.
- Prime editors: These advanced editors offer greater precision and versatility in making edits, reducing off-target effects compared to traditional CRISPR-Cas9.
Ethical Implications and Societal Impact
CRISPR-Cas9 technology, while offering immense potential benefits across various fields, presents profound ethical and societal challenges that require careful consideration. Its power to alter the human genome raises complex questions about responsibility, equity, and the very definition of human nature. The potential for both immense good and significant harm necessitates a robust and ongoing dialogue involving scientists, ethicists, policymakers, and the public.
Germline Editing and its Ethical Considerations
Germline editing, the modification of genes in reproductive cells (sperm or eggs) or early embryos, carries unique ethical weight. Unlike somatic cell editing, which affects only the individual undergoing treatment, germline edits are heritable, meaning the changes are passed down to future generations. This raises concerns about unforeseen consequences and the potential for unintended harm extending far beyond the initial recipient. The possibility of introducing heritable changes without fully understanding the long-term effects raises serious ethical questions about our right to manipulate the human gene pool. For instance, altering genes associated with desirable traits might inadvertently create unforeseen health problems in subsequent generations.
Potential Misuse of CRISPR Technology
The potential for misuse of CRISPR technology is a significant concern. Its relative ease of use and accessibility raise the possibility of its application in unethical or harmful ways. Examples include its use for non-therapeutic genetic enhancements, aiming to create “designer babies” with specific physical or cognitive traits. This could exacerbate existing social inequalities by making genetic enhancements accessible only to the wealthy, creating a genetic divide. Furthermore, the technology could be misused for malicious purposes, such as developing genetically modified pathogens for bioterrorism. The ease of CRISPR’s application also makes it more accessible to those without proper training or ethical considerations.
Societal Impact of CRISPR Technology
CRISPR technology has the potential to revolutionize various aspects of society. On the positive side, it promises breakthroughs in treating genetic diseases, improving crop yields to address food security, and developing more accurate diagnostic tools. However, negative impacts are also possible. The aforementioned genetic divide, coupled with concerns about job displacement due to automation driven by CRISPR-enhanced agriculture, could lead to social unrest and economic inequality. The potential for unintended environmental consequences from genetically modified organisms also needs careful evaluation and mitigation. For example, the introduction of CRISPR-modified crops could disrupt existing ecosystems if the modified organisms outcompete native species or if unintended gene flow occurs.
Responsible Innovation and Regulation
Responsible innovation and stringent regulation are crucial for harnessing the benefits of CRISPR technology while mitigating its risks. This necessitates a multi-faceted approach involving international collaborations to establish ethical guidelines and safety standards, rigorous oversight of research and clinical trials, and public engagement to foster informed decision-making. Transparency in research, open access to data, and independent ethical review boards are essential components of a responsible innovation framework. Furthermore, educational initiatives are needed to increase public understanding of CRISPR technology and its implications, empowering individuals to participate meaningfully in the ongoing societal dialogue surrounding its use. The potential benefits are enormous, but realizing them responsibly requires a proactive and globally coordinated effort.
CRISPR-Based Drug Discovery
CRISPR-Cas systems, initially renowned for their gene-editing capabilities, have rapidly emerged as powerful tools in drug discovery. Their precision and versatility allow researchers to efficiently identify drug targets, validate their function, and screen for potential therapeutic compounds, significantly accelerating the drug development process compared to traditional methods. This section explores the diverse applications of CRISPR technology in drug discovery, focusing on target identification and validation, functional genomics screening, and a comparative analysis with established approaches.
Target Identification and Validation Using CRISPR
CRISPR technology significantly enhances target identification and validation by enabling precise gene disruption or modulation. Researchers can systematically knock out or knock down individual genes within a cell line and observe the resulting phenotypic changes. If the disruption of a specific gene leads to a desired therapeutic effect (e.g., reduced tumor growth, improved cellular function), that gene becomes a strong candidate drug target. This approach is far more efficient than traditional methods, which often rely on indirect evidence or lengthy biochemical assays. For example, researchers might use CRISPR to systematically knock out genes in cancer cells to identify genes whose disruption leads to cell death, thereby pinpointing potential targets for anticancer drugs. The subsequent validation process involves confirming the target’s role using complementary approaches, such as CRISPR-mediated gene activation or the use of specific inhibitors.
CRISPR-Based Functional Genomics Screening
Functional genomics screening using CRISPR allows for high-throughput analysis of gene function on a genomic scale. Libraries containing thousands of guide RNAs (gRNAs), each targeting a different gene, can be used to systematically disrupt genes within a cell population. Researchers can then assess the impact of each gene disruption on a specific phenotype, identifying genes that influence disease processes or drug responses. This approach is particularly useful for identifying novel drug targets and understanding complex biological pathways. For instance, a CRISPR-based screen could be used to identify genes involved in drug resistance, paving the way for the development of combination therapies to overcome resistance. This type of screening significantly accelerates the discovery of potential therapeutic targets, surpassing the capabilities of traditional, more laborious approaches.
Workflow for CRISPR in Drug Development
A typical workflow for CRISPR-based drug development might proceed as follows: 1) Target Identification: Conducting a CRISPR screen to identify genes influencing a disease-related phenotype. 2) Target Validation: Using CRISPR to confirm the role of the identified target in disease pathogenesis through gene knockout or knockdown experiments. 3) Drug Discovery: Screening for compounds that modulate the activity of the validated target, potentially using CRISPRi (CRISPR interference) to systematically reduce the expression of the target and assess the impact on the phenotype. 4) Lead Optimization: Refining the identified compounds to improve their potency, selectivity, and pharmacokinetic properties. 5) Preclinical Studies: Conducting in vitro and in vivo studies to evaluate the efficacy and safety of the drug candidate. 6) Clinical Trials: Initiating clinical trials to assess the drug’s safety and efficacy in humans. This structured approach leverages CRISPR’s precision at each stage, streamlining the drug development process.
Comparison of CRISPR and Traditional Drug Discovery Methods
Traditional drug discovery methods often involve lengthy and laborious processes, including target identification through extensive biochemical and genetic studies, followed by extensive screening of chemical libraries. CRISPR technology offers a significant advantage by accelerating the target identification and validation stages. CRISPR-based functional genomics screens allow for a high-throughput, systematic analysis of gene function, surpassing the capabilities of traditional methods. The ability to precisely manipulate gene expression provides a more direct and efficient approach to validating targets compared to indirect methods relying on correlations. While traditional methods remain relevant, the integration of CRISPR technology offers a significant advancement in speed, efficiency, and precision in drug discovery, leading to faster and potentially more effective therapeutic development.
CRISPR and Cancer Therapy
CRISPR-Cas9 technology, with its precise gene-editing capabilities, holds immense promise for revolutionizing cancer treatment. Its ability to target specific genes implicated in cancer development and progression offers a potential pathway to more effective and personalized therapies. While still largely in the research phase, several approaches are under investigation, demonstrating the significant potential of this technology.
CRISPR-Based Cancer Therapies Under Investigation
Several CRISPR-based strategies are currently being explored in preclinical and clinical trials for various cancer types. These approaches primarily focus on either directly targeting cancer cells or enhancing the efficacy of existing cancer therapies. Examples include targeting genes that drive tumor growth, disrupting cancer cell signaling pathways, and engineering immune cells to more effectively recognize and destroy cancer cells.
Challenges of Using CRISPR for Cancer Treatment
Despite its potential, the application of CRISPR in cancer therapy faces significant hurdles. Off-target effects, where CRISPR edits unintended genomic locations, remain a major concern, potentially leading to harmful consequences. Efficient delivery of the CRISPR-Cas9 system to cancer cells is another challenge, as it requires overcoming the complexities of tumor microenvironments and minimizing damage to healthy tissues. Furthermore, the heterogeneity of cancer cells, with their diverse genetic profiles, complicates the development of universally effective CRISPR-based therapies. The immune response to the introduced CRISPR components also needs careful consideration.
Combining CRISPR with Other Cancer Therapies, Crispr technology
The synergistic potential of combining CRISPR with other established cancer therapies is a promising area of research. For example, CRISPR could be used to enhance the effectiveness of immunotherapy by engineering immune cells (like T cells) to more efficiently target and eliminate cancer cells. Similarly, combining CRISPR with chemotherapy or radiotherapy could potentially improve their efficacy while reducing side effects by making cancer cells more sensitive to these treatments or by targeting genes responsible for drug resistance. This combination approach holds the key to overcoming some of the limitations of individual therapies.
Types of CRISPR-Based Cancer Therapies
| Therapy Type | Mechanism of Action | Examples |
|---|---|---|
| Gene Knockout | Disrupting the function of cancer-driving genes. | Targeting oncogenes (e.g., KRAS, MYC) involved in cell growth and proliferation. |
| Gene Correction | Repairing mutated tumor suppressor genes. | Correcting mutations in genes like p53, which are involved in cell cycle regulation and apoptosis. |
| Immune Cell Engineering | Modifying immune cells to enhance their anti-tumor activity. | Engineering CAR T-cells to express chimeric antigen receptors with improved specificity and efficacy. |
| Oncolytic Virus Modification | Engineering viruses to specifically target and destroy cancer cells. | Modifying oncolytic viruses to enhance their tumor-targeting capabilities and improve their therapeutic index. |
Advancements and Future Directions
CRISPR technology, while already revolutionizing various fields, continues to evolve at a rapid pace. Recent advancements are not only improving its precision and efficiency but also expanding its potential applications across medicine, agriculture, and beyond. Understanding these advancements and the challenges ahead is crucial for realizing the full potential of this groundbreaking technology.
Recent advancements in CRISPR technology have focused on enhancing its specificity, reducing off-target effects, and developing more efficient delivery systems. These improvements are paving the way for safer and more effective therapeutic applications. Furthermore, the development of novel CRISPR-associated proteins (Cas) with unique properties is expanding the range of genomic targets and applications. For instance, the discovery of CasX and CasY, smaller and potentially more versatile Cas proteins than the widely used Cas9, opens up new possibilities for gene editing in confined spaces, such as within the confines of a cell nucleus.
Improved Specificity and Reduced Off-Target Effects
The initial iterations of CRISPR-Cas9 systems suffered from off-target effects, where the Cas9 enzyme could inadvertently cut DNA at unintended locations. This limited the clinical applicability of the technology. However, significant progress has been made in improving specificity through various strategies. These include the development of high-fidelity Cas9 variants with enhanced target recognition, the use of guide RNA modifications to improve binding affinity and specificity, and the implementation of paired nickases to minimize off-target cleavage. These advancements have dramatically reduced off-target effects, making CRISPR technology safer for therapeutic use. For example, the development of high-fidelity Cas9 variants like eSpCas9(1.1) has demonstrated a significant reduction in off-target cuts compared to the wild-type Cas9.
Novel CRISPR-Associated Proteins (Cas)
The discovery of new Cas proteins with different properties has broadened the scope of CRISPR technology. Beyond Cas9, researchers have identified several other Cas proteins, each with unique characteristics, such as different target recognition sequences or the ability to perform different types of gene editing. These diverse Cas proteins offer greater flexibility in targeting specific genes and performing various gene editing functions, including base editing and prime editing, which offer greater precision than simple gene cutting. The exploration and engineering of these novel Cas proteins are expanding the possibilities for CRISPR applications.
Advanced Delivery Systems
Efficient delivery of CRISPR-Cas systems to target cells or tissues remains a significant challenge. Early methods relied on viral vectors, which can have limitations in terms of immunogenicity and target cell tropism. Recent advancements include the development of non-viral delivery methods, such as lipid nanoparticles and nanoparticles conjugated with cell-penetrating peptides, that offer improved safety and targeting capabilities. These advancements are critical for translating CRISPR-based therapies into clinical practice. For instance, lipid nanoparticles have shown success in delivering CRISPR-Cas components to specific organs in animal models, suggesting their potential for future human applications.
Potential Future Applications of CRISPR Technology
The future applications of CRISPR technology are vast and span multiple fields. Beyond gene therapy for genetic diseases, CRISPR holds immense potential in areas such as:
- Developing novel cancer therapies by targeting cancer-specific genes or enhancing the immune system’s ability to fight cancer cells.
- Creating disease-resistant crops and livestock through precise gene editing in agriculture.
- Developing advanced diagnostic tools for rapid and accurate disease detection.
- Engineering microorganisms for biofuel production and environmental remediation.
Key Challenges for Wider Adoption
Despite the significant advancements, several challenges remain before CRISPR technology can be widely adopted. These include:
- Ensuring complete safety and minimizing off-target effects.
- Developing efficient and targeted delivery systems for various tissues and organs.
- Addressing ethical concerns and regulatory hurdles associated with gene editing.
- Developing robust and accessible diagnostic tools to monitor the effects of CRISPR therapies.
Timeline of Major Milestones in CRISPR Technology Development
| Year | Milestone |
|---|---|
| 2012 | First demonstration of CRISPR-Cas9 for targeted gene editing in bacteria. |
| 2013 | Successful application of CRISPR-Cas9 in mammalian cells. |
| 2015 | First clinical trials using CRISPR-based therapies begin. |
| 2017 | Development of base editing and prime editing technologies. |
| 2020 | Continued advancements in improving specificity and reducing off-target effects. |
| 2023 | Growing exploration of novel Cas proteins and advanced delivery systems. |
Intellectual Property and Commercialization
The CRISPR-Cas9 gene-editing technology has sparked a revolution in biotechnology, leading to a complex and fiercely contested intellectual property (IP) landscape. This has significant implications for the commercialization of CRISPR-based products and services, presenting both substantial opportunities and considerable challenges for companies and researchers alike. The key players involved in this IP battle are striving to secure patents and licenses, shaping the future of this transformative technology.
The current intellectual property landscape surrounding CRISPR technology is characterized by a multitude of overlapping and sometimes conflicting patents. This stems from the independent development of CRISPR-Cas9 by multiple research groups, leading to a series of patent applications filed around the world. The most prominent dispute involves the Broad Institute and the University of California, Berkeley, each claiming ownership of crucial aspects of the technology. These legal battles have significantly impacted the licensing landscape, creating uncertainty for companies seeking to commercialize CRISPR-based products. Furthermore, the ongoing development of new CRISPR systems and related technologies further complicates the IP landscape, with ongoing patent applications and licensing agreements constantly evolving.
Challenges and Opportunities in Commercializing CRISPR-Based Products
Commercializing CRISPR-based products presents a unique set of challenges and opportunities. The high cost of research and development, coupled with the stringent regulatory hurdles involved in bringing gene-editing therapies to market, represent significant barriers to entry. However, the potential market for CRISPR-based therapeutics, diagnostics, and agricultural products is enormous, creating a strong incentive for companies to navigate these complexities. The long timelines associated with clinical trials and regulatory approvals require substantial upfront investment and a long-term perspective. Furthermore, public perception and ethical concerns surrounding gene editing can impact market acceptance and regulatory approval pathways. Conversely, the potential for revolutionary advancements in healthcare and agriculture creates a substantial opportunity for early movers to establish market dominance and reap significant financial rewards. The successful commercialization of CRISPR-based products will depend on a strategic balance of scientific innovation, robust IP protection, and effective regulatory navigation.
Business Models for Commercializing CRISPR Technologies
Several distinct business models are employed for commercializing CRISPR technologies. Some companies focus on developing and licensing CRISPR-based tools and technologies to other research institutions and companies. This model allows for broad dissemination of the technology while generating revenue through licensing fees and royalties. Other companies are developing and commercializing their own CRISPR-based products, such as therapeutic agents or diagnostic tools. This requires significant investment in research and development, clinical trials, and regulatory approvals but offers the potential for higher profit margins. A third model involves collaborations and partnerships between academic institutions, biotechnology companies, and pharmaceutical companies. These collaborations combine the expertise and resources of different organizations, accelerating the development and commercialization of CRISPR-based products. Strategic alliances also help mitigate the risks associated with high-cost development and lengthy regulatory processes.
Examples of Companies Commercializing CRISPR-Based Products or Services
Several companies are actively involved in commercializing CRISPR-based products or services. Intellia Therapeutics, for example, is focused on developing CRISPR/Cas9-based therapies for various diseases. CRISPR Therapeutics is another prominent player in the therapeutic space, working on treatments for beta-thalassemia and sickle cell disease. Editas Medicine is another company pursuing therapeutic applications, focusing on genetic diseases affecting the eye. In the agricultural sector, companies like Pairwise are utilizing CRISPR technology to develop improved crop varieties with enhanced traits. These examples highlight the diverse applications of CRISPR technology and the different approaches companies are taking to commercialize its potential. The continued growth and diversification of this field will likely lead to the emergence of even more innovative business models and commercial products in the years to come.
CRISPR and Infectious Diseases
CRISPR-Cas systems, initially recognized for their role in bacterial adaptive immunity, have emerged as powerful tools in the fight against infectious diseases. Their ability to precisely target and modify DNA offers unprecedented opportunities for developing novel antiviral therapies and diagnostic tools. However, the rapid evolution of many viruses presents significant challenges that need to be addressed for successful implementation.
CRISPR’s potential in combating viral infections stems from its ability to target viral genetic material. This can be achieved through several mechanisms, including directly disrupting the viral genome, inhibiting viral replication, or enhancing the host’s immune response. The precision of CRISPR allows for targeted intervention without affecting the host’s own DNA, minimizing off-target effects.
CRISPR-Based Antiviral Therapies
The development of CRISPR-based antiviral therapies is a rapidly advancing field. One promising approach involves designing CRISPR systems to target essential viral genes, thereby disrupting the virus’s life cycle. Another strategy focuses on targeting viral integration sites in the host genome, preventing viral persistence and reactivation. Furthermore, CRISPR can be employed to enhance the host’s immune response by modifying immune cells to better recognize and eliminate infected cells. Research is ongoing to explore the efficacy and safety of these approaches in preclinical and clinical settings. For instance, studies are exploring the use of CRISPR to target specific regions of the HIV genome, aiming to eliminate the virus from infected cells.
Challenges in Targeting Rapidly Mutating Viruses
A major hurdle in applying CRISPR against viruses, particularly RNA viruses like influenza and HIV, is their high mutation rate. These viruses constantly evolve, generating new variants that can escape CRISPR targeting. This necessitates the development of CRISPR systems that can adapt to these mutations, either through the use of broadly targeting CRISPR molecules or by employing strategies that anticipate and counteract viral mutations. One strategy under investigation is the development of CRISPR systems that target highly conserved regions of the viral genome, which are less prone to mutation. Another approach involves using multiple CRISPR guide RNAs to target different regions of the viral genome simultaneously, making it less likely that the virus will escape targeting through mutation.
Hypothetical CRISPR-Based Antiviral Strategy for Influenza A Virus
Consider a hypothetical CRISPR-based antiviral strategy for Influenza A virus (IAV). IAV is a rapidly mutating RNA virus responsible for seasonal flu epidemics. A potential approach would involve engineering a CRISPR-Cas system delivered via a modified adeno-associated virus (AAV) vector. The AAV would carry a Cas9 enzyme and multiple guide RNAs targeting highly conserved regions within the IAV genome, specifically focusing on genes essential for viral replication, such as polymerase genes or hemagglutinin (HA) genes. The AAV would be administered to infected individuals, delivering the CRISPR system to the respiratory cells. Upon expression, the Cas9 enzyme would use the guide RNAs to precisely target and cleave the IAV genome, thereby inhibiting viral replication and reducing viral load. This strategy would ideally need to be adapted to target the prevalent IAV strains circulating during a particular flu season, highlighting the challenge of keeping pace with viral evolution. The use of multiple guide RNAs targeting different conserved regions would improve the robustness of the system against viral escape mutations. Preclinical studies in animal models would be crucial to assess the efficacy and safety of such a strategy before clinical trials could be initiated.
Concluding Remarks
CRISPR technology’s impact extends far beyond the laboratory, promising transformative advancements in medicine, agriculture, and diagnostics. While ethical concerns and potential risks require careful navigation, the ongoing research and development in this field suggest a future where CRISPR’s potential benefits are harnessed responsibly to address significant global challenges. Continued refinement of techniques, coupled with robust regulatory frameworks, will be crucial in realizing the full potential of this remarkable technology.
CRISPR technology, a revolutionary gene-editing tool, relies on precise targeting and manipulation of DNA sequences. The computational power needed to design and analyze CRISPR experiments is substantial, often requiring sophisticated algorithms and high-performance computing, which are areas of expertise within the field of computer systems technology. Ultimately, advancements in both fields are mutually beneficial, accelerating the development and application of this powerful gene editing technology.
CRISPR technology, a revolutionary gene-editing tool, holds immense potential for various applications. Research into its ethical implications and practical uses is ongoing at many institutions, including tennessee technological university , where scientists are likely exploring novel applications of this powerful technology. Further advancements in CRISPR promise to reshape fields like medicine and agriculture in the coming decades.





